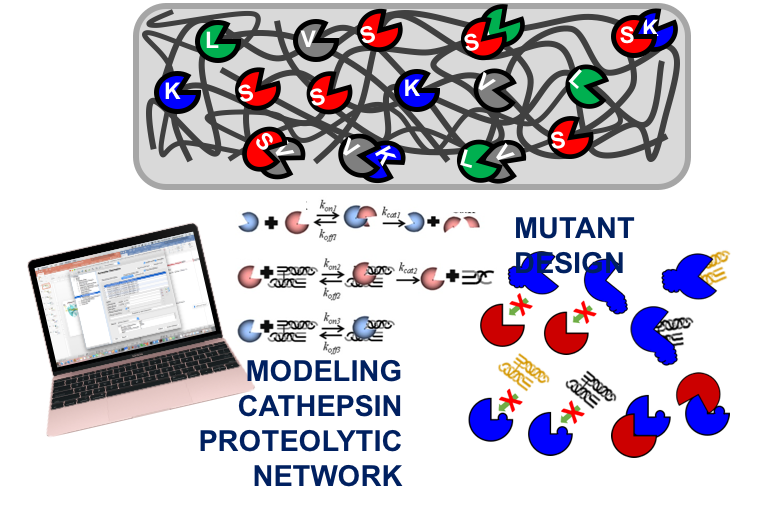
Reassessing enzyme kinetics: considering protease-as-substrate interactions in proteolytic networks.
Proteases are enzymes that degrade proteins and play a major role in cellular homeostasis. One such class, cysteine cathepsins, contain the most powerful mammalian collagenase and elastase. When studied in combination, there are protease-on-protease interactions, cathepsin cannibalism interactions, that ultimately reduce the pool of active protease in a system. Cysteine cathepsins are lysosomal proteases involved in extracellular matrix remodeling and are often found upregulated in tissue destructive diseases such as cancer, osteoporosis, atherosclerosis, rheumatoid arthritis, and tendinopathy. Since cysteine cathepsins are so potent and involved in many different pathologies, they have been a key target for pharmaceutical companies, but all have failed phase II and III clinical trials due to off-target side effects. We hypothesized that an incomplete understanding of how individual cathepsin species interact with each is causing problems in appropriately dosing inhibitors for treatment. Part of my thesis work focused on using computational techniques to tease apart these cathepsin cannibalism interactions in a proteolytic network consisting of cathepsins K, L, S, gelatin and elastin, as well as design and test mutant cathepsins to resist these interactions. I developed a software program called PACMANS to identify putative sites on the cathepsin sequence highly susceptible to cleavage by other cathepsins. Using site-directed mutagenesis, mutations were made at these putative sites and resulted in cathepsins mutants resistant to cannibalism by other cathepsins.
- Ferrall-Fairbanks MC, Kieslich CA, Platt MO. “Reassessing enzyme kinetics: Considering protease-as-substrate interactions in proteolytic networks.” Proceedings of the National Academy of Sciences of the United States of America, 2020 Feb 11; 117(6):3307-3318. doi: 10.1073/pnas.1912207117 PMID: 31980525
- Ferrall-Fairbanks MC, Barry ZT, Affer M, Shuler MA, Moomaw EW, Platt MO. “[PACMANS: A Bioinformatically Informed Algorithm to Predict, Design, and Disrupt Protease-on-Protease Hydrolysis]().” Protein Science, 2017 Apr; 26(4):880-890. doi: 10.1002/pro.3113 PMID: 28078782
- Ferrall MC, Affer M, Platt MO. “Kinetic Models of Cathepsin Protease Interactions to Improve Pharmaceutical Inhibition of Extracellular Matrix Degradation.” Oral presentation at the 2015 Annual Meeting of the Society for Mathematical Biology, June 30-July 3, 2015, Atlanta, Georgia, USA
- Ferrall-Fairbanks MC, Kieslich CA, Platt MO. “Reassessing enzyme kinetics: considering protease-as-substrate interactions in proteolytic networks.” Under Review